Do you search for 'how to write electrochemical cell reactions'? You will find all the information on this section.
Table of contents
- How to write electrochemical cell reactions in 2021
- Cell notation to equation
- Cell notation for redox reaction
- How to write cell notation
- Lead copper electrochemical cell
- Applications of electrochemical cells
- Electrochemical cell diagram labeled
- Electrochemistry cell
How to write electrochemical cell reactions in 2021
 This image demonstrates how to write electrochemical cell reactions.
This image demonstrates how to write electrochemical cell reactions.
Cell notation to equation
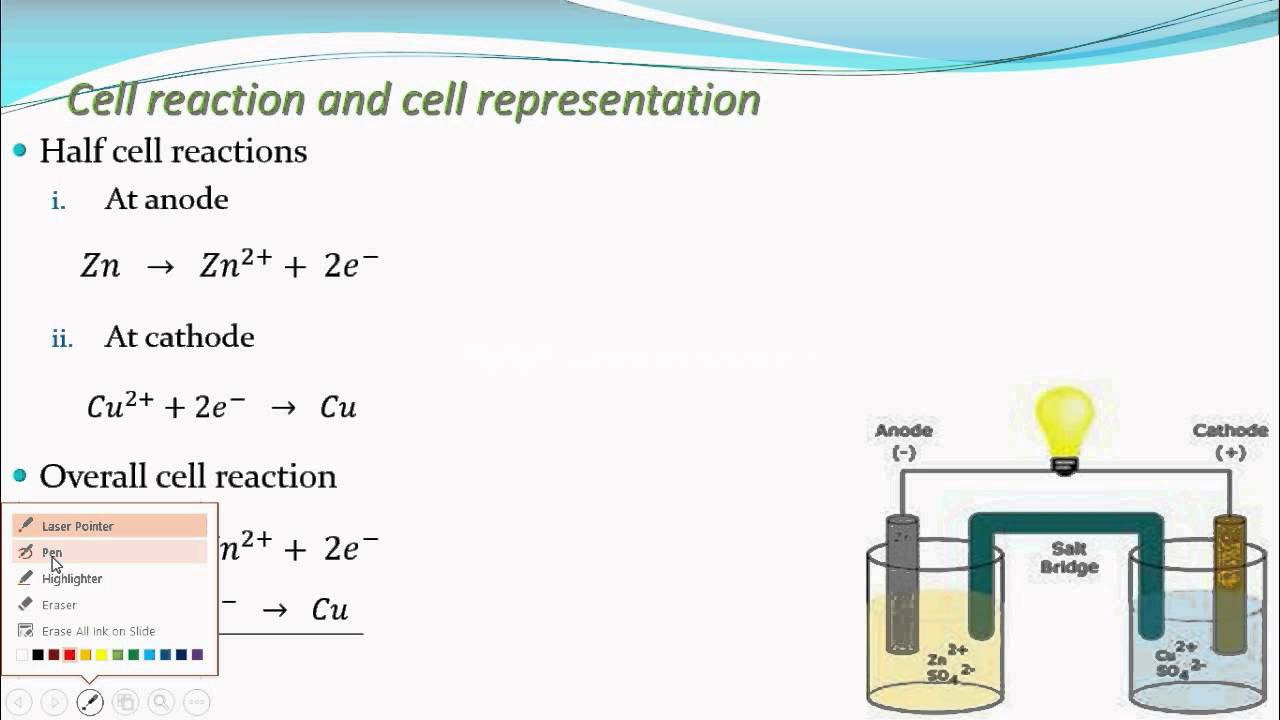 This image representes Cell notation to equation.
This image representes Cell notation to equation.
Cell notation for redox reaction
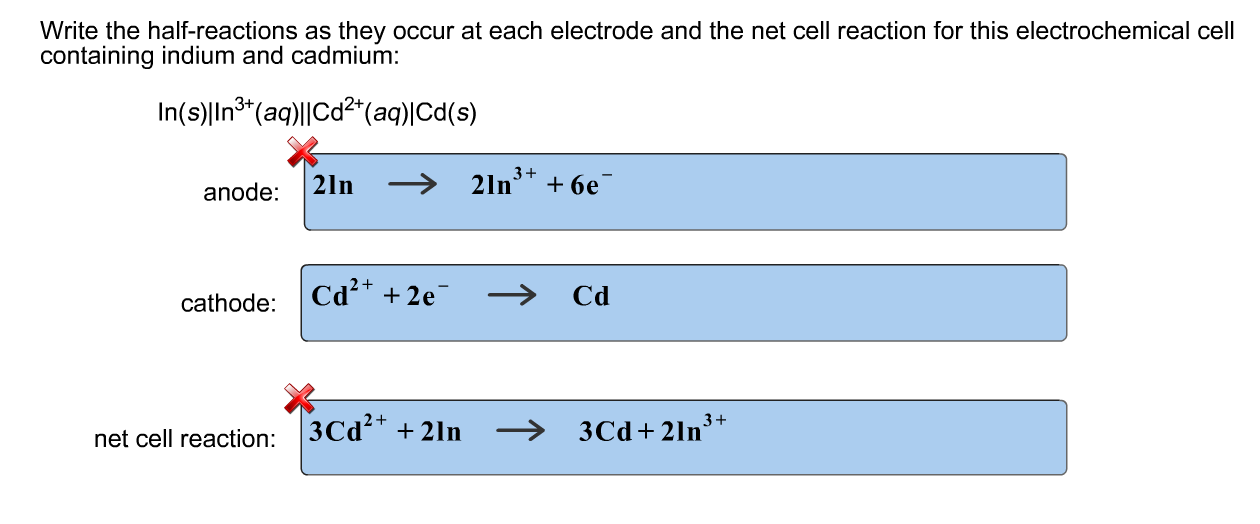 This image illustrates Cell notation for redox reaction.
This image illustrates Cell notation for redox reaction.
How to write cell notation
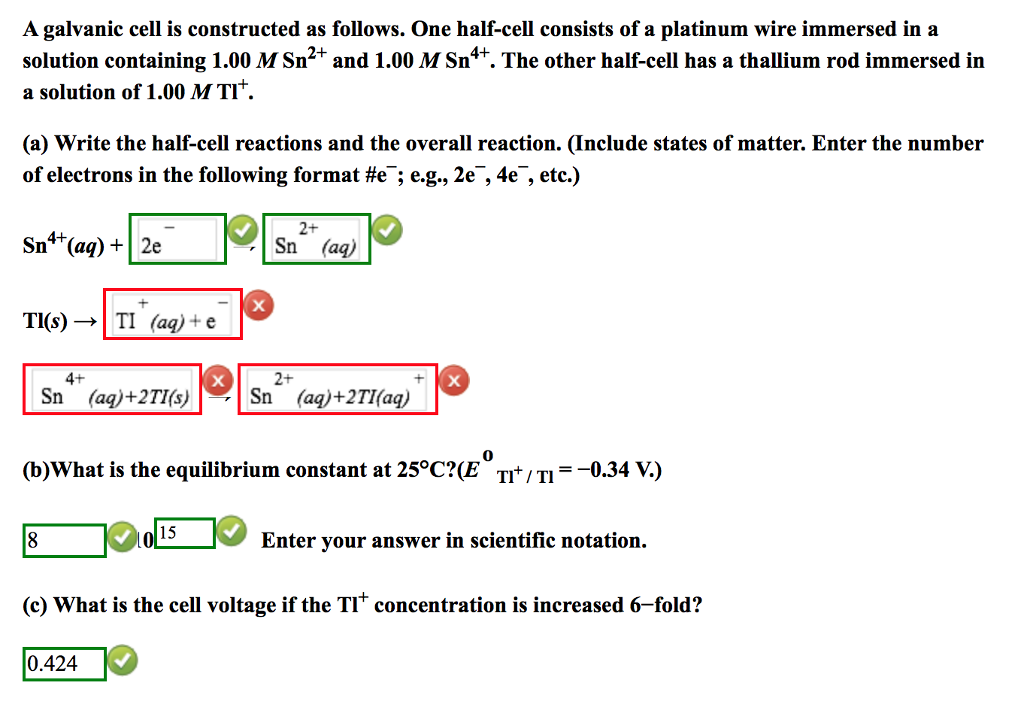 This picture demonstrates How to write cell notation.
This picture demonstrates How to write cell notation.
Lead copper electrochemical cell
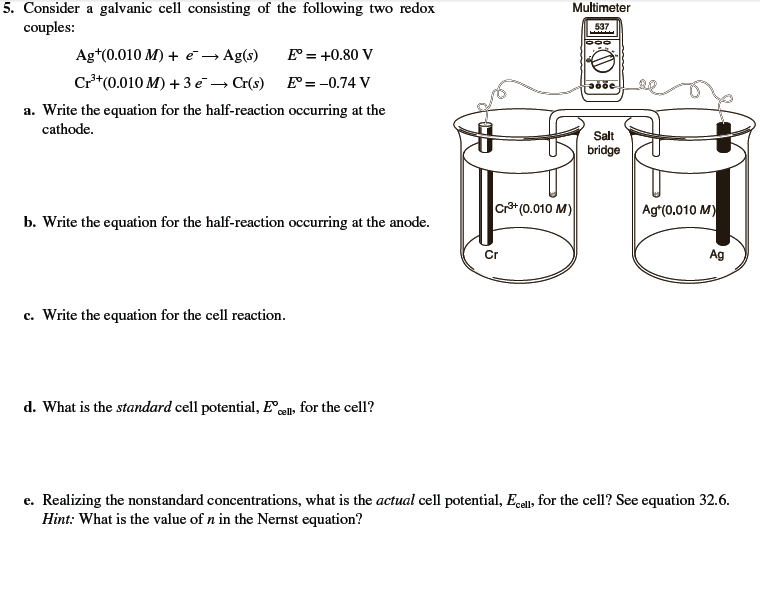 This image demonstrates Lead copper electrochemical cell.
This image demonstrates Lead copper electrochemical cell.
Applications of electrochemical cells
 This image shows Applications of electrochemical cells.
This image shows Applications of electrochemical cells.
Electrochemical cell diagram labeled
 This image representes Electrochemical cell diagram labeled.
This image representes Electrochemical cell diagram labeled.
Electrochemistry cell
 This image representes Electrochemistry cell.
This image representes Electrochemistry cell.
What happens when you turn an electrochemical reaction around?
Turning the reaction around doesn't change the relative strengths of the oxidizing or reducing agents. The magnitude of the potential must remain the same. But turning the equation around changes the sign of the cell potential, and can therefore turn an unfavorable reaction into one that is spontaneous, or vice versa.
How are cell potentials calculated in electrochemical chemistry?
Cell Notation. Recall that standard cell potentials can be calculated from potentials E0cell for both oxidation and reduction reactions. A positive cell potential indicates that the reaction proceeds spontaneously in the direction in which the reaction is written.
How are the half cells of an electrochemical reaction connected?
One half-cell contains the anode and an electrolyte containing the same metal cations. The other half-cell contains the cathode and an electrolyte containing the same metal cations. These half-cells are connected by a salt-bridge and the electrodes are connected through an external circuit.
How to write the equation for an electrochemical reaction?
Remember that for an electrochemical cell the standard cell notation is: Give the anode and cathode half-reactions. Write the overall equation for the chemical reaction. Give the direction of the current in the external circuit.
Last Update: Oct 2021